Reproduction & dispersal
Dispersal
Once spores or vegetative propagules have been produced they need to be released and dispersed if new plants are to develop. There is considerable variation in sporophyte anatomy – in both the spore capsule and, when present, the supporting seta. All aspects of sporophyte structure have some influence on how the spores get out and are dispersed. The aim of this section is to show you many of the ways in which dispersal can happen and, for spore dispersal, the roles played by sporophyte anatomy. We'll look first at the ways in which spores are dispersed and then at vegetative propagules.
Spores
Most bryophytes rely on wind for spore dispersal. The vast majority of species have small spores, typically with diameters of 5 to 50 micrometres, a micrometre being a thousandth of a millimetre. An example at the other extreme is the moss genus Archidium, with spore diameters mostly in the range 100-200 micrometres, but as low as 50 micrometres, in Archidium dinteri (known only from southern Africa) and up to 300 micrometres, almost a third of a millimetre, in Archidium ohioense. The latter is a widespread species, known from Africa, Asia, North America, the West Indies and New Caledonia![]() .
.
Small spores can be carried considerable distances by the wind. Even very light breezes, virtually imperceptible to a person, can easily waft the smaller spores away. Wind dispersal gets more difficult with spores of about 50 micrometre diameter so that Archidium spores, for example, are too heavy for wind to be an effective dispersal agent. Strong winds may certainly move them short distances, just as sand grains can be blown about, but they would be carried more easily by water. In addition, such spores may well be dispersed when mixed up with mud that is picked up by animal feet. Finally, there is a small number of moss species in which insects are the main agents of spore dispersal.
Regardless of how the spores are dispersed they must first get out of the capsule. The capsule may develop a well-defined mouth, through which the spores can escape. If the capsule lacks such a mouth it may split along well-defined lines of weakness (the dehiscence lines) or break open irregularly to expose the spores, for further dispersal by wind or some other agency.
In mosses the majority of species have capsules with well-defined mouths but you will also find species where the capsules break irregularly and the capsules in a couple of genera have dehiscence lines. The majority of liverwort species have capsules with dehiscence lines but there are also species with disintegrating capsules. Hornwort capsules have one or two dehiscence lines. There is variation in the structure of mouths and the ways of splitting. We will now look more closely at the three ways in which capsules can open.
Disintegrating capsules
In a few moss genera the capsule disintegrates and examples of this are Acaulon, Archidium, Ephemerum and Pleuridium. The spores either tumble out of the broken capsules or may be washed away, for example by flowing surface water after rain. The genus Pleurophascum (confined to the southern coast of Western Australia, Tasmania and the south island of New Zealand) also appears to have disintegrating capsules, though there are still some unanswered questions about this genus. The species Pleurophascum grandiglobum (right) is endemic to Tasmania, and a Tasmanian bryologist has reported frequently seeing partly eroded or grazed spore capsules. The identity of the creature responsible for the grazing is unknown as is the role, if any, that this creature plays in spore dispersal. The capsules of the endemic New Zealand species Pleurophascum ovalifolium are globose when immature but (unlike those of Pleurophascum grandiglobum) collapse to a discoid shape when mature. The capsules of this species seem to take much longer to develop than do those of the Tasmanian species and they also appear to be longer lasting. One New Zealand bryologist has speculated that the entire spore capsule, when close to maturity but still globose, may function as a dispersal agent. The globose capsule contains much air and could easily float on water and would presumably disintegrate, and release spores, at some distance from the parent plant. In this connection it is worth noting Pleurophascum ovalifolium characteristically occurs in very wet sites![]() .
.
In the complex thallose liverwort genus Riccia the spore capsules are embedded in the thallus. Riccia is a widespread and commonly seen genus, with many species. When mature the capsule and overlying thallus disintegrate, leaving the spores exposed within a cup-like depression. The spores in this genus are commonly 60-80 micrometres in diameter and too large to be easily wind-dispersed, but water could wash them away. Moreover, as the thallus keeps growing at its tip, the older parts will progressively disintegrate. So eventually any spores that have been unable to disperse from those cup-like depressions will be left loose on the soil, where they may germinate or disperse more easily.
Fossombronia , a simple thallose liverwort genus, is also widespread with many species. A mature spore capsule is raised on a flimsy, translucent seta and the capsule wall breaks irregularly into small plate lets, which fall away to expose the spore mass.
At first sight it might appear that complex thallose liverwort genus Targionia has spore capsules that split. This photo ![]() shows several plants with mature spore capsules. At the ends of the green, strap-like thalli you can see what look like open, black clam-shells. You could be excused thinking that these are black capsules that have opened to release the spores. In fact those black "shells" are not part of the capsule, though they do surround the developing capsule and form a protective pouch. The capsule itself has thinner walls that break. Targionia is commonly found on soil in habitats that periodically become very dry. Spores may at times escape as the pouch decays. However, there is another, more common process. As conditions dry the thallus closes, the sides rolling inwards, towards the long central axis. Instead of being a green strap, a thallus now looks like a black cord. The black scales that were originally on the underside of the thallus show well after the inrolling. At the same time that black "cord" arches up from the ground to raise the pouch, which opens to expose the spores and elaters from the already ruptured capsule
shows several plants with mature spore capsules. At the ends of the green, strap-like thalli you can see what look like open, black clam-shells. You could be excused thinking that these are black capsules that have opened to release the spores. In fact those black "shells" are not part of the capsule, though they do surround the developing capsule and form a protective pouch. The capsule itself has thinner walls that break. Targionia is commonly found on soil in habitats that periodically become very dry. Spores may at times escape as the pouch decays. However, there is another, more common process. As conditions dry the thallus closes, the sides rolling inwards, towards the long central axis. Instead of being a green strap, a thallus now looks like a black cord. The black scales that were originally on the underside of the thallus show well after the inrolling. At the same time that black "cord" arches up from the ground to raise the pouch, which opens to expose the spores and elaters from the already ruptured capsule ![]() . Thus, even though the spore capsule develops close to the soil, a drying atmosphere raises the pouch (and hence the spores) a centimetre or two into the air where they have a greater chance of being caught and dispersed by breezes
. Thus, even though the spore capsule develops close to the soil, a drying atmosphere raises the pouch (and hence the spores) a centimetre or two into the air where they have a greater chance of being caught and dispersed by breezes![]() .
.
Splitting capsules
If we take the point where the capsule is attached to a seta (or, in the absence of a seta, to the gametophyte) as the "south pole" and the opposite point as the "north pole", then the dehiscence lines are oriented north-south like lines of longitude. The number of dehiscence lines varies between species. When a capsule splits along dehiscence lines there are two possibilities – the splitting goes all the way from the "south pole" to the "north pole" or it stops short. In the first case a mature capsule opens out in a number of arms to give a somewhat star-like appearance. This is what occurs in the majority of liverwort species. Usually there are four dehiscence lines and hence four arms in the open capsule. Within the capsules there are elaters as well as spores. Elaters are tubular cells with spiral thickenings that often help in spore release. Elaters do not work in the same way in all species. The elaters may twist or untwist with changes in humidity, or spring suddenly when released from tension. In such cases the movement of the elaters helps fling the spores a short distance into the air where air currents can pick them up and carry them away. In some liverworts the elaters in the spore capsules move about little, if at all, and play little, if any, role in spore release. There's more about the workings of elaters in the ELATERS SECTION.
Dehiscing capsules may split in the way just described. The other possibility, noted earlier, is that the splitting stops short of the "north pole". In such a case the capsule cannot open out fully, since the arms are joined at their apices. In two closely-related moss genera, Andreaea and Andreaeaobryum, the mature capsule has four or more lines of weakness. In many species of these genera the lines of weakness do not extend to the apex of the capsule. As the mature capsule begins to dry out the capsule shrinks in length. The outer capsule cells shrink less than the inner ones and this causes the capsule to bow out so that slit-like gaps form along the dehiscence lines and the spores can fall out through those gaps. If the capsule is moistened the gaps close up, but will re-open when dry again. Note that a dehiscing liverwort capsule, once open, stays open and does not close up if moistened. When the capsules of the mosses mentioned here are dry and showing the gaps, they look a bit like old-style lanterns - so giving these mosses the common name of Lantern Mosses.
Hornwort spore capsules are generally of a long, tapering form, ![]() the exception being the genus Notothylas in which the capsules are relatively short. At maturity hornwort capsules split, along their length, along either one or two dehiscence lines. The splitting starts near, but not at, the apex of the capsule. Depending on whether the capsule has one or two lines of weakness, it opens via one or two slits. The spores near the apex mature first, then the ones a little lower down, then the ones further down and so on. As the spores lower down mature, so the slit (or slits) extend downward, keeping pace with the maturing spores. The capsule becomes twisted as it dries and the slits open to allow spores to be blown out by breezes. Under moist conditions the capsule untwists and the slits close up to block spore release
the exception being the genus Notothylas in which the capsules are relatively short. At maturity hornwort capsules split, along their length, along either one or two dehiscence lines. The splitting starts near, but not at, the apex of the capsule. Depending on whether the capsule has one or two lines of weakness, it opens via one or two slits. The spores near the apex mature first, then the ones a little lower down, then the ones further down and so on. As the spores lower down mature, so the slit (or slits) extend downward, keeping pace with the maturing spores. The capsule becomes twisted as it dries and the slits open to allow spores to be blown out by breezes. Under moist conditions the capsule untwists and the slits close up to block spore release![]() .
.
Capsules with mouths
In the great majority of mosses the mature spore capsules have well-defined mouths through which the spores are released, The mouths are formed at the end of the spore capsule opposite the point at which the capsule is attached to the seta or, if there is no seta, opposite the point at which the capsule is attached to the gametophyte. During the development of the spore capsule (covered in more detail in the SPOROPHYTE DEVELOPMENT SECTION) the mouth is covered by a firmly attached lid (or operculum). That attachment must be broken if the spores are to get out![]() .
.
In Sphagnum the process is typically explosive, with spores and operculum shot off simultaneously. As the mature capsule begins to dry it shrinks, compressing the air inside. Eventually the internal pressure becomes enough to force the operculum off and shoot the spores into the air where breezes will pick them up. In most mosses the process is not explosive. Rather, the operculum is released fairly gently and the spores are released over an extended period. Even in Sphagnum spore release is not always explosive. This genus is most often found in bogs. Sometimes a rise in water levels may leave mature capsules submerged and then the explosive process cannot take place, since it relies on the drying out of the capsule. In such circumstances the capsule falls off its supporting stalk and the columella decays to leave a small hole at the base of the capsule. Spores can escape through that hole. Another possibility is for the spores to germinate while still in the attached capsule and then burst the capsule as the germinating plants expand.
Eccremidium is a predominantly Australian moss genus. They are soil mosses with gametophytes no more than a few millimetres tall and the spores are fairly large, from 50 to 140 micrometres in diameter. The capsules are spherical to pear-shaped with the operculum occupying about half the capsule. This is unusual, with the opercula in other genera occupying very little of the capsule. Once the spores of an Eccremidium have matured the operculum falls off, leaving a smooth-rimmed mouth that is relatively large, often with a diameter equal to that of the spore capsule. In three of the six Eccremidium species known from Australia the seta holding the capsule is bent over so that the capsule is held with the mouth angled downwards. The large spores would find it easy to fall out of the large, smooth-rimmed mouth. Even in species where the mouth is not angled downwards some disturbance of the capsule (for example by wind, water or animal) would probably be enough to shake the spores out.
Here are some plants of the genus Bryum ![]() , each with a green, immature capsule atop a seta. The capsules are also held so that the mouths face downward and they will keep this orientation as the capsules mature and turn from green to brown. In each capsule the operculum is relatively small but things still seem simple enough. Once the operculum has come off surely the spores will fall out. However, a closer look shows that things aren't quite that simple. In the majority of mosses (including the genus Bryum) the mouth is lined with teeth of some sort. These are called the peristome teeth by some writers
, each with a green, immature capsule atop a seta. The capsules are also held so that the mouths face downward and they will keep this orientation as the capsules mature and turn from green to brown. In each capsule the operculum is relatively small but things still seem simple enough. Once the operculum has come off surely the spores will fall out. However, a closer look shows that things aren't quite that simple. In the majority of mosses (including the genus Bryum) the mouth is lined with teeth of some sort. These are called the peristome teeth by some writers ![]() (with the rim around the mouth being the peristome), while others simply use the word peristome to mean a toothed mouth. Peristome teeth may move in response to changes in humidity, either closing or opening the mouth to stop or allow spore release. There is variation in structure of peristome teeth and there are genera which lack peristome teeth. You've already seen Eccremidium as an example of the latter and Sphagnum is another. Given the explosive nature of spore release in Sphagnum, it is clear that such teeth would have no function - and would in fact hinder spore release.
(with the rim around the mouth being the peristome), while others simply use the word peristome to mean a toothed mouth. Peristome teeth may move in response to changes in humidity, either closing or opening the mouth to stop or allow spore release. There is variation in structure of peristome teeth and there are genera which lack peristome teeth. You've already seen Eccremidium as an example of the latter and Sphagnum is another. Given the explosive nature of spore release in Sphagnum, it is clear that such teeth would have no function - and would in fact hinder spore release.
We'll finish this section with some more detailed examples of the ways in which capsules work.
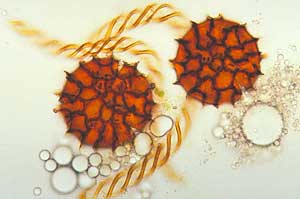
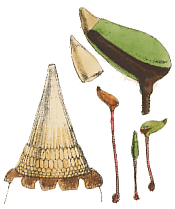
The peristome teeth in the moss genus Dawsonia are long and threadlike (right), so making the capsule look as though it has a tuft of white hairs around the mouth. In immature sporophytes the capsules are held upright. However the mature capsules are relatively large (about a centimetre long) and will have turned to be held horizontally so that they present a large surface area to falling raindrops. Any raindrop (or runoff from overhead plants) that hits the upper side of the capsule momentarily depresses the capsule wall and so (analogous to a puffball fungus) forces a puff of spores out between those threadlike teeth. Capsules in the genera Buxbaumia and Diphyscium also present relatively large surface areas, though the capsules are smaller than those of Dawsonia, often no more than half a centimetre in length. Once again capsules struck by falling raindrops puff out spores. In the case of Buxbaumia the capsules orient themselves so that the mouth is pointed towards the highest light intensity. Where the light intensity is highest, the obstructions are least. Puffing the spores in that direction would increase their chances of clearing surrounding obstacles and dispersing further away. The painting at the top of this page shows views of Buxbaumia aphylla. On the upper right you can see a close-up of a capsule, in reality about five millimetres long. On the lower left is a much closer view of the peristome and on the right are some whole plants. Between the capsule and peristome pictures is the calyptra, which covers the very young sporophyte.
In a small number of moss species (in the family Splachnaceae) spore dispersal is primarily by dung- or carrion-loving insects. These mosses grow on the dung of various animals and occasionally on old animal carcases. Theoretically the spores are small enough to be wind-dispersed but they are sticky and clump together, so ruling out wind dispersal. The capsules are often highly modified, coloured to attract insects and producing insect-attracting chemicals. Here is a description of the spore release process in some of these mosses. At maturity the spore capsule sheds the operculum. In dry conditions the capsule walls shrink, forcing the peristome teeth to bend back so as to finish up turned down against the outside wall of the spore capsule. At the same time the shrinkage of the capsule leads to the columella extending beyond the capsule mouth. The tip of the columella is coated with the sticky spores. Insects, attracted to the capsule, will almost inevitably pick up clumps of the sticky spores. Being dung- or carrion-loving insects they'll naturally visit other carcases or droppings and so carry spores exactly to the sorts of substrates that these mosses exploit. In moist conditions the capsule swells (so bringing the columella back within the capsule) and the peristome teeth fold back over the mouth and spore release stops.
Schistostega pennata, a widespread Northern Hemisphere moss, is another species with sticky spores. It's not in the family Splachnaceae and also seems to be without any features (such as colour or chemicals) that would attract a specific type of organism to act as a dispersal agent.
Vegetative propagules
There are many agents which can help in the dispersal of vegetative propagules. To take the example closest to home, think of humans. In the VEGETATIVE REPRODUCTION SECTION there was brief mention of fragmentation of mosses in lawns ![]() by a lawn mower. Such fragments could then be easily carried further afield by that lawn mower. Alternatively, suppose that a gardener is raking fallen leaves off that lawn. The rake may well catch and pull out some strands of this creeping moss - which fall elsewhere as the gathered leaves are being removed. Both the mown fragments and the raked fragments are capable of generating new plants in the right habitats. Thinking of taking a walk through a grassy paddock? One bryologist found fragments of the moss Thuidiopsis furfurosa had adhered to his socks when he'd walked through a grassy, New Zealand meadow. This moss is brittle in the dry state, so fragments could easily break off and attach to fur, feathers - or socks. Most people are well aware of the annoying burrs, grass seeds and so on that are readily picked up by socks. In some grassy areas various species of creeping mosses may grow fairly luxuriantly and, with the surrounding grasses for support, grow to ankle height where they can get caught by socks. On a bush walk you will have brushed against some shrubs or had a lie down. Later that day, as you're about to get in your car for the trip home, you brush bits of rubbish from your jumper – leaves, seeds, twigs and fragments of moss or liverwort. How far have you carried those fragments – 10 metres or 10 kilometres? You've just acted as a very effective disperser of vegetative propagules
by a lawn mower. Such fragments could then be easily carried further afield by that lawn mower. Alternatively, suppose that a gardener is raking fallen leaves off that lawn. The rake may well catch and pull out some strands of this creeping moss - which fall elsewhere as the gathered leaves are being removed. Both the mown fragments and the raked fragments are capable of generating new plants in the right habitats. Thinking of taking a walk through a grassy paddock? One bryologist found fragments of the moss Thuidiopsis furfurosa had adhered to his socks when he'd walked through a grassy, New Zealand meadow. This moss is brittle in the dry state, so fragments could easily break off and attach to fur, feathers - or socks. Most people are well aware of the annoying burrs, grass seeds and so on that are readily picked up by socks. In some grassy areas various species of creeping mosses may grow fairly luxuriantly and, with the surrounding grasses for support, grow to ankle height where they can get caught by socks. On a bush walk you will have brushed against some shrubs or had a lie down. Later that day, as you're about to get in your car for the trip home, you brush bits of rubbish from your jumper – leaves, seeds, twigs and fragments of moss or liverwort. How far have you carried those fragments – 10 metres or 10 kilometres? You've just acted as a very effective disperser of vegetative propagules![]() .
.
Other vertebrates
Apart from humans many other animals, in their normal activities, may help disperse bryophyte fragments. A German study, published in 2001, found 106 bryophyte fragments on 9 wild boar and 25 roe deer. Those fragments represented 12 species. The bristly coats of wild boar picked up more fragments than the sleeker coats of the roe deer. The wallowing and rooting habits of wild boar make it very easy for them to pick up bryophyte fragments. Deer, when lying down, could pick up fragments on their coats. To study this the researchers used a "dummy deer", made of a deer skin filled with foam plastic. This dummy was placed on its stomach on the forest floor. In addition the researchers mimicked a deer's wallowing motion by gently rocking the dummy from side to side a few times and also by pushing it back and forth with gentle pressure. Then the dummy's skin was cleaned of all adhering plant fragments and those were studied. The whole process was done 300 times, at random points in the forest study site, and the dummy yielded 51 bryophyte fragments. Both the boar and the deer had also picked up fragments in their hooves. Wild boar in particular, with their bristly coats and ranging up to 5 kilometres per day in European forests, may well be significant dispersers of forest bryophytes. This study was a small one, with a very small number of animals examined and there are some interesting unanswered questions. For example, how representative of other deer and boar were these 34 animals? Furthermore, in the course of a day an animal could pick up fragments, drop some of them, pick up some more, drop some more and so on. What is the total number of fragments moved per animal per day? However, as the researchers stated, the subject of animals and bryophyte fragments has not been studied systematically![]() .
.
Th is study has pointed out some interesting possibilities and shown that further study would be worthwhile. Moreover, think of what could be happening in an Australian setting - a potaroo digging for native truffles, a wombat pushing through undergrowth, two possums fighting on a tree branch, an arid area red kangaroo creating a shallow soil scrape. In each of those situations bryophytes could be fragmented and lodge in animal fur.
Fragments of the cosmopolitan moss species Bryum argenteum have been found on the feet of Antarctic skuas and penguins. Presumably as these birds land on or walk over a mossy patch fragments occasionally get scuffed loose and then get picked up unintentionally. Dense bryophyte cushions create stable micro-habitats for various invertebrates. You can often see insectivorous birds pecking or scraping such cushions to get at those invertebrates. In the process fragments of various sorts may be produced and even picked up accidentally. Various birds deliberately pick up strands of trailing mosses and use them to help camouflage nests. <<GET PIC OF NEST>> If the conditions are right those gathered strands will continue to grow on the nest.
In Queensland the Spectacled Flying Fox (Pteropus conspicillatus) is potentially occasional disperser of bryophytes. Viable fragments have been collected from the droppings of this bat and grown on in the laboratory in artificial culture. That still leaves open the question of what is the fate of the dung-embedded fragments in the wild, but presumably at least a small proportion would grow into new plants. Rather than deliberately choosing to eat bryophytes the evidence suggests that the bats swallow fragments while grooming![]() .
.
Invertebrates
Numerous invertebrates live in bryophyte colonies or move through them. Various invertebrates eat bryophytes, lay their eggs on them or excavate burrows in them. Some caddis fly larvae use bryophyte fragments on their larval cases. During all such activities small fragments could be accidentally released and of course a bryophyte fragment on a discarded larval case may continue growing if that larval case is discarded in a suitable habitat. The widespread moss species Fissidens fontanus (which you may also see referred to as Octodiceras fontanum) is found on rocks in and beside streams. In Northern Europe it is also found on dead or live freshwater clams of the species Anodonta cygnea. These clams may move occasionally and so help disperse the moss. Leptodictyon riparium is another moss that is typically found on streamside rocks but which has also been reported on molluscs. Liverworts or mosses have been found on Papuan weevils and Brazilian harvestmen. It is likely that in the course of their roaming these invertebrates could lose pieces of bryophytes, for example during fights. The bryophytes involved are also found on rocks or plants, so the species are not reliant on the invertebrates![]() .
.
Some of the vegetative propagules described in the VEGETATIVE REPRODUCTION SECTION are very easily dislodged. Even the disturbance caused by a small invertebrate moving along a bryophyte colony may be enough to loosen a tiny gemma or a fragile branch tip. The dislodged propagules could simply fall onto the immediate surrounds, but some could be picked up by the passing invertebrate on its furry or bristly body, to be dislodged or groomed off later. The northern hemisphere moss Schistostega pennata produces gemmae on the protonemal stage (which develops immediately after spore germination and is covered in the LIFE CYCLE SECTION). These gemmae are rounded at the end that is attached to the protonema, but long and tapering at the opposite end. That tapering end is extremely sticky in fresh material and mites have been seen with the gemmae of this moss attached to their legs. Undoubtedly various other invertebrates would also pick up such sticky gemmae. It is interesting to note that the spores of Schistostega pennata are also sticky![]() .
.
Inanimate agents
Inanimate forces may also break pieces off bryophytes. Storms may break and blow away bryophyte covered twigs. If those twigs land in a suitable habitat the bryophytes can continue growing in their new location. From time to time streamside erosion will break bryophyte colonies, with the stream then carrying any pieces further afield. Once again, if the pieces land in suitable habitats they'll continue growing. Strong winds may cause fragmentation, particularly in areas with little in the way of windbreaks. In desert, alpine and polar regions (where even low shrubbery is sparse to absent) winds may blow unchecked and for long periods. Furthermore, wind-blown sand or snow crystals add to the abrasive effects of wind alone, a sustained wind is drying and dry bryophytes are usually brittle. Putting all these factors together, we have ideal conditions for fragmentation. In many cold regions periods of freezing alternate with periods of thawing and such freeze/thaw cycles could also cause fragmentation.
In a study of a site on Bathurst Island, in the Canadian Arctic, the researchers estimated that there were at least 4,000 propagules per cubic metre of granular snow near the end of the yearly melt. The particular snow bed being studied had melted completely during the previous summer. Therefore all fragments would have been deposited during the winter immediately before the investigators did their sampling. They also tried growing about 900 fragments back at the laboratory and over a four and a half month period 12% showed new growth. Naturally, there will always be questions as to how accurately a laboratory result represents what happens in nature. However, the study does show that a large number of viable propagules could be produced annually on Bathurst Island. At the other end of the world, windblown vegetative propagules have also been studied from the Antarctic and sub-Antarctic areas. On Macquarie Island or at Casey station in Antarctica researchers found gemmae, deciduous shoots, leaves, leaf fragments and stem fragments with attached leaves. Many of these produced new growth in laboratory experiments![]() .
.
![An Australian Government Initiative [logo]](/images/austgovt_brown_90px.gif)