
Bird Orchids
Small deciduous terrestrial orchids that reproduce from seed and also form clonal colonies. Leaves 2, opposed, nearly equal, usually prostrate; margins wavy; underside grey-glaucous. Inflorescence single-flowered, arising centrally where the leaves join; peduncle thin, fleshy; pedicel short. Flowers mostly horizontal, usually greenish or reddish. Sepals with short to long cylindrical clubs, sometimes of a different colour to the blade. Dorsal sepal narrow, incurved close to the column. Lateral sepals fused at the base, projected forwards then decurved beneath the labellum, narrow, parallel or divergent. Petals membranous, reflexed against the ovary. Labellum stiffly hinged at the base. Labellum lamina widest towards the apex, the upper surface covered with shiny calli, these variously shaped, coloured and arranged in a formation that resembles an insect such as an ant or wingless wasp. Column narrow, incurved over the labellum, narrowly to broadly winged.
Similar Genera
Significant Generic Characters
Deciduous terrestrials; leaves 2, subequal, opposed; flowers relatively small, suberect to horizontal, greenish or reddish; sepals with cylindrical osmophores; petals reflexed against the ovary; labellum stiffly hinged; labellum lamina widest distally, mainly rhomboid-trapeziform in shape; calli variously shaped, sessile or stalked, shiny, black or colourful, in a strongly insectiform arrangement; column wings narrow to broad.
Size and Distribution
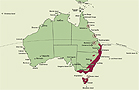
A genus of about 10 species endemic in eastern Australia. Latitudinally in Australia Chiloglottis is distributed from about 17°16' S on the Atherton Tableland, Queensland, to about 43°30' S in southern Tasmania. State occurrence: Queensland, New South Wales, Australian Capital Territory, Victoria, Tasmania.
Ecology
Species of Chiloglottis are terrestrial orchids which are commonest and most diverse in the temperate regions of southeastern Australia, with a single species extending into the wet tropics of northeastern Queensland. They range from the coast to the inland side of the Great Dividing Range and from near sea level into montane areas of about 1500 m alt. in the Barrington Tops, New South Wales and about 1600 m alt. on Mt Bartle Frere in northeastern Queensland. They grow in well-drained soils in such habitats as rainforest margins, wet sclerophyll forest, open forest, woodland, heathy forest, coastal scrub and heathland. Some species also colonise pine plantations.
Biology
Pollination: Species of Chiloglottis are pollinated by sexual deceit involving the males of specific species of thynnine wasps in a pseudocopulation arrangement. Pollinator -choice experiments carried out by Colin Bower show conclusively that within its natural range each Chiloglottis species is pollinated by a single thynnine species in a highly specialised relationship. The wasps are attracted to the flowers by odours (allomones) which mimic the pheromones released by female wasps to attract the males for mating purposes. These allomones are produced by terminal osmophores on the sepals and also by the labellum calli. In all species of Chiloglottis the calli are arranged on the labellum such that they bear a remarkable resemblance to an insect with its head aligned towards the base of the flower. This arrangement, termed pseudofemale, may play a visual role in attracting the male thynnine wasps when they are in close proximity to the flower. The male wasp lands on the flower, usually the stiffly hinged labellum, and grasps the head of the pseudofemale while facing into the flower. Contact is made with the anther and/or stigma while the wasp is probing the labellum margins with its abdomen or attempting to fly off with the decoy female (Bower 2001).
No species of Chiloglottis is autogamous and apomixis is unknown in the genus.
Reproduction: Reproduction in Chiloglottis is by seed and all species grow in clonal colonies that result from the production of daughter tubers on the end of stolonoid roots. Seed dispersal occurs 8-12 weeks after pollination. After pollination and prior to seed dispersal the pedicel undergoes a remarkable transformation, thickening and elongating up to 30 cm in length. This elongation presumably aids seed dispersal since these orchids often grow in sheltered, shrubby habitats.
Seasonal Growth: Species of Chiloglottis occur in areas with a seasonal climate and the plants have periods of active growth and dormancy.
Flowering: Species of Chiloglottis flower in late summer and autumn. The buds emerge along with the leaves and the plants sometimes flower while the leaves are not fully developed.
Hybrids: Natural hybridisation is very rare within Chiloglottis.
Fire: Flowering in Chiloglottis is generally inhibited by summer bushfires at least for the first season until the microhabitats are restored. A significant proportion of plants are killed by very hot fires. No species is known to be fire dependant, although flowering in C. reflexa is enhanced by summer fires.
Derivation
Derived from the Greek cheilos, lip, and glottis, mouth of the windpipe, referring to the resemblance of the labellum and its callus to the human windpipe.
Botanical Description
Perennial geophytic herbs, sympodial. Plants glabrous. Roots filamentous. Tubers ovoid, solitary, fleshy, naked; replacement tubers absent; daughter tubers formed on the end of slender stolonoid roots. Stem erect, short, unbranched, with membranous cataphylls at each node. Trichomes absent. Leaves 2, basal, subequal, opposed, petiolate; lamina mostly narrowly elliptic, convolute in bud; margins usually undulate; petiole grooved adaxially. Venation anastomosing. Inflorescence racemose, 1-flowered, erect, terminal. Peduncle fleshy, with no sterile bracts. Floral bract foliaceous, sheathing. Pedicel short, erect. Ovary elongate, ribbed, glabrous, straight. Flowers resupinate, suberect to horizontal, dull coloured, pedicellate. Dorsal sepal free, broader than the lateral sepals, incurved over the column, with a terminal osmophore. Lateral sepals united at the very base, much narrower than the dorsal sepal, parallel or divergent, with short to long, terete terminal osmophores. Petals free, broader than the lateral sepals, usually reflexed against the ovary. Labellum free, attached by a small claw to the underside of the column foot, hinged but capable of only limited movement, markedly dissimilar in size and shape to the sepals and petals, ecalcarate. Labellum lamina unlobed, more or less rhombic-trapeziform in shape, tapered to a narrow base. Spur absent. Callus variable, consisting of calli in various shapes, sizes and colour in a strongly insectiform arrangement that occupies some or most of the labellum ventral surface; calli stalked or sessile. Nectar absent. Column lacking free filament and style, incurved. Column wings narrow, ventral, extending from base to apex. Column foot present, reduced to a vestigial bump. Pseudospur absent. Anther terminal, 4-celled, persistent, basifixed, erect, rostrate. Pollinarium absent. Pollinia 4, curved, flat, mealy, yellow; pollen in tetrads. Viscidium absent. Rostellum ventral. Stigma entire, circular, concave. Capsules dehiscent, glabrous, erect; sepals and petals persistent; peduncle not elongated in fruit; pedicel considerably elongated and thickened in fruit. Seeds numerous, light coloured, winged.
Taxonomy
Chiloglottis has recently been split into 2 segregate genera, Simpliglottis (Szlachetko 2001) and Myrmechila (Jones and Clements 2005).
Nomenclature
Chiloglottis R.Br., Prodr. 322 (1810). Type species: Chiloglottis diphylla R.Br.
Infrageneric Taxa: No infrageneric taxa are recognised.
References
Backhouse, G. and Jeanes, J. (1995). The Orchids of Victoria. Miegunyah Press, Carlton, Victoria.
Bishop, T. (1996). Field Guide to the Orchids of New South Wales and Victoria. University of New South Wales Press, Sydney.
Bower, C.C. (1996). Demonstration of pollinator-mediated reproductive isolation in sexually deceptive species of Chiloglottis (Orchidaceae: Caladeniinae). Aust. J. Bot. 44: 15-33.
Bower, C.C. (2001). Pollination of Chiloglottis in Pridgeon, A.M., Cribb, P.J., Chase, M.W. and Rasmussen, F.N. (eds), Genera Orchidacearum, Vol. 2. Oxford University Press.
Carr, G.W. (1991). New taxa in Caladenia R.Br., Chiloglottis R.Br. and Gastrodia R.Br. (Orchidaceae) from south-eastern Australia. Indigenous Flora and Fauna Association, Miscellaneous Paper No. 1.
Clements, M.A. (1989). Catalogue of Australian Orchidaceae. Austral. Orch. Res. 1: 1-160.
Curtis, W.M. (1979). The Student’s Flora of Tasmania, Part 4A. Government Printer, Hobart.
Entwisle, T.J. (1994) in Walsh, N.G. and Entwisle, T.J. (1994). Flora of Victoria, vol. 2. Inkata Press, Melbourne.
Harden, G. (ed.) (1993). Flora of New South Wales, vol. 4. New South Wales University Press, Sydney.
Jones, D.L. (1991). New taxa of Australian Orchidaceae. Austral. Orch. Res. 2: 37-44.
Jones, D.L. (1998). Contributions to Tasmanian Orchidology - 3: A taxonomic review of Chiloglottis R.Br. in Tasmania. Austral.Orch. Res. 3: 61-71.
Nicholls, W.H. (1969). Orchids of Australia. Thomas Nelson, Melbourne.
Ross, E.M. and Jones, D. (1989), in Stanley, T.D. and Ross, E.M., Flora of South-eastern Queensland, vol. 3. Queensland Department of Primary Industries.
Rupp, H.M.R. (1943). The Orchids of New South Wales. Australian Medical Publishing Coy, Sydney.
Szlachetko, D.L. (2001). Genera et Species Orchidalium. 1. Polish Bot. J. 46(1): 11-26.
Willis, J.H. (1970). A Handbook to Plants in Victoria vol. 1 (2nd ed.). Melbourne University Press, Carlton.