 Chemistry
Chemistry
Chemistry after the 1860s
There are two pages on this website dealing with the major historical episodes from the first century of 'chemical' lichenology. The early years were the subject of CHEMISTRY IN THE 1860s and the current page continues the story. Much of the information is taken from the paper cited in the following reference button. About 10 of its pages are devoted to a historical background![]() .
.
The later 1800s
The first history page discussed the introduction and enthusiastic use of chemical testing by William Nylander and William Leighton. The reagents used were iodine solutions (signified by I in modern literature), bleaching powder (signified by C) and potassium hydroxide (signified by K). The first page also noted the considerable criticism of chemical testing by William Lauder Lindsay, but despite those criticisms various other lichenologists followed Nylander's lead and used the chemical tests as aids to identification. However, there were also those who refused to use chemistry in any way and the next few decades saw much debate about the role of chemistry in the study of lichens. While those decades saw more people adopt the use of chemical tests as an aid in identification it remained a fact that the reasons for the colour changes remained unknown. Which compounds reacted with I, K or C to produce those colours? What were their chemical structures? Whenever a reagent produced a particular colour change, was it always reacting with the same lichen compound or could more than one lichen compound produce the same colour change with the reagent in question? Questions such as these would need to be answered if there were to be a solid foundation to the use of chemistry in lichen taxonomy. To jump ahead a bit, research in the 20th century showed that a colour change in a spot test indicated only which class of compounds were involved, rather than a specific compound. For example, if application of K produces a yellow colour, followed soon by a change to red, then one or more o-hydroxy aromatic aldehydes are present but a method much more discriminating than a spot test is needed to identify which aldehyde is involved in any particular case.
Amongst the later 19th century's many recordings of the presence or absence of colour changes in numerous species some interesting observations about micro-crystals were published by E. Bachmann in 1887. For example, he noted that the striking K-induced yellow-to-red reaction just mentioned was even more amazing when viewed under a microscope since a vast number of rusty-red to blood-red, needle-like crystals crystallised out of the yellow solution. To the naked eye this mass of crystals was just a homogenous, blood-red spot. Bachmann noted further that under a polarizing microscope the crystals glowed a vivid golden-yellow. Very shortly there will be more about the use of micro-crystals for lichen identification![]() .
.
The 20th century
Friederich Wilhelm Zopf (1846-1909) was the first to carry out extensive chemical analyses and the year 1907 saw the publication of his book Die Flechtenstoffe in chemischer, botanischer, pharmakologischer und technischer Beziehung. Flechten is the German word for lichens and the composite word Flechtenstoffe literally means "lichen substances". The book contained descriptions of over 150 chemical compounds found in lichens and the science of lichen chemistry can be said to start with Zopf's work. Little was still known about the actual structures of many of those compounds but Zopf's work did give a sounder basis for the use of chemistry in the taxonomy of lichens. That's not to say that chemistry was universally embraced. Some still refused to allow chemistry a role but, contrariwise, by the late 1920s a few lichenologists had gone to the other extreme, using chemistry indiscriminately to define species and paying no heed to anatomical features. Such actions tended to bring chemical methods into disrepute.
Further fundamental work on chemical structures was carried out by Yasuhiko Asahina (1880-1975) and his colleagues in Japan in the 1930s and later. In 1934 Asahina introduced another test reagent: p-phenylenediamine (symbolised as P). As well as devising the P test Asahina also introduced a micro-crystallization technique for routine identification of secondary metabolites. In this technique the compounds are first extracted from a lichen fragment with acetone. Then the acetone is evaporated and the residue is re-crystallized from a suitable solvent. All this is a fairly simple process in that no specialized equipment is needed, though it still takes some skill to carry out the several steps in the process successfully. Different compounds crystallize in different shapes or colours. By comparing the crystallized product from a mystery lichen with photographs of crystals of known substances it was often possible to identify the substance in the mystery lichen. The crystallization technique allowed better recognition of secondary metabolites, with the ability to detect microgram amounts, than did spot tests though one drawback was the failure to detect joint occurrences of some metabolites. Nevertheless the micro-crystallization technique was extremely useful until superseded by chromatographic methods and was taken up by various European and North American lichenologists. Asahina's careful studies did much to rehabilitate chemistry from the indiscriminate excesses mentioned two paragraphs ago. His detailed chemical studies convinced him that there were times when chemical differences alone were sufficient to differentiate species, but this was still not a universally accepted view point![]() .
.
From the early 1950s paper chromatography had been applied to the analysis of lichen metabolites and later developments such as thin-layer chromatography (TLC) and high-performance liquid chromatography (HPLC) allowed even more accurate analyses of lichen compounds and these techniques replaced the micro-crystal method. These chromatographic tools allowed the detection of very small concentrations of metabolites so gave a more detailed picture of the chemistries of the different species. If chemistry were to be useful then it was important that the production of secondary metabolites not be dependent on the age of the thallus. The first published investigation of this question appeared in 1958. The authors had analysed 138 different-aged specimens of Lasallia papulosa and showed that the internal chemistry was constant and so showed chemistry to be a valid tool in lichen taxonomy![]() .
.
|
The latter half of the 20th century saw a great increase in the number of organic chemists who chose to investigate the chemical complexity of lichens, for lichens are interesting biological associations that present a variety of intellectual challenges to organic chemists. By the end of the 20th century the secondary metabolites of over 5,000 lichen species had been studied, that was about a third of the species then known. Getting to that level of knowledge had been an international effort with the researchers based in numerous countries. While much of the analysis has been done by the various chromatographic methods noted above other methods of chemical analysis were also used and you can see those listed in the box to the right![]() .
.
The debate about the role of chemistry continued through the 20th century, with some denying chemistry any role in differentiating species but perhaps allowing it a role in distinguishing variation within a species. Thus terms such as subspecies, chemical strain, chemical variety (or chemovar) or chemical race were proposed. Others argued that no single feature be it chemical, ecological, some structural characteristic and so on) had an absolute value. Rather, in each case, it was necessary to look at all the information, and in particular the correlations between the different lines of evidence, before coming to any conclusions as to the weight to be given to chemistry. That view was put very pithily by the American lichenologist William Culberson who (101 years after Lindsay's dismissal of chemistry) wrote the following:
The use of chemistry in species-level taxonomy by some lichenologists has been attended by a controversy that, like many matters taxonomic, seems to have less to do with science than with emotion. The notion that in living organisms variation that can be seen (morphology) is necessarily more fundamental than variation that cannot (chemistry) is axiomatic. Yet I am not aware that this pompous dictum has ever been proved to be true; I very much doubt that it is, and I would guess that such mottoes passed off as the wisdom to channel taxonomic thought are nothing but a hollow defense of tradition. If one sees the role of taxonomy to be the construction of natural systems based on all relevant data from studies on the systematics of organisms, then an a priori rejection or relegation of chemical variations to a preconceived rank (subspecies, "chemovarietas", etc.) is philosophically indefensible
.
The present & the future
Chemistry has been used in lichenology for over a century and has been shown to be useful in both identification and taxonomy. Moreover, chemical differences are now recognized as being very useful not only in helping to differentiate different species within a genus, but also as playing a key role in distinguishing various genera. In the 1860s chemistry was a new tool and whenever a new tool or method has been introduced into taxonomy two things are almost inevitable - the enthusiastic (sometimes over-enthusiastic) espousing of the new by some taxonomists and the doubts (sometimes hypercritical) expressed by others. Doubt in itself is neither surprising nor a problem since any new approach must be able to show that it can produce useful results and so the new approach must be thoroughly tested. Another thing that usually happens whenever a new approach is introduced is that it gives results that agree with some aspects of the existing taxonomy but cast doubts on other aspects and chemistry has certainly done that. Such doubts invite a fresh look at all the features of the species in question.
As noted early on this page often, when chemical differences have been found in, say, a species other differences can also be found (for example in morphology, ecology or geographical distribution, to give a few examples) which correlate with the chemical differences with the total evidence justifying the division of that 'species' into two or more distinct species. In some cases the other differences had not previously been noted whereas in others they had been noted but not thought to be significant.
In relatively recent years another new tool, molecular analysis of DNA, has been used to study lichens but such analyses are still in their early days. The results thus far have supported some of the earlier taxonomic ideas (including some involving chemistry) but cast doubt on others. So it is again a case of looking afresh at those cases where the molecular evidence has raised questions. Molecular analysis is being used to construct evolutionary histories and once such histories have been constructed it can be interesting to see how non-genetic features relate to such a history. For example, suppose that analysis shows species A and B to have similar DNA while that of species C differs significantly from the DNA of both A and B. On the basis of that evidence A and B are more closely related to each other than either is to C and so you could summarise those results in an evolutionary diagram such as the following.
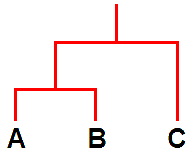
It's a bit like a family tree. The species A and B are like brother and sister while C is a cousin to both A and B. Following that analogy, apart from A, B and C, there are also ancestors. There is an ancestor that gave rise to all three but there is also an ancestor that gave rise to just A and B. All of that has been based on DNA analysis. Now suppose that C has X and Y as major lichen compounds while A and B have X as a major lichen compound but Y is absent. In this very simplistic scenario the chemical facts correlate perfectly with the DNA evidence and when such correlations occur they invite hypotheses about the evolutionary history of chemical products. In the present case one hypothesis would be that the ancestor to all 3 species produced X and Y while the ancestor that gave rise to just A and B had lost the ability to produce Y after a genetic change. That's by no means the only possible hypothesis and in any real study there would be additional evidence (and possibly complications) but this simple picture shows how the combination of DNA analysis and chemical facts could shed light on the evolution of biosynthetic pathways in lichens.
The authors of a paper published in 2001 gave the following as an example of a realistic question along the lines of the ideas outlined in the previous paragraph:
... are the genes for the biosynthesis of common phenolic acid units such as orsellinic acid, which is a precursor to many nonlichen and lichen products, derived from a common ancestor or have different genes for this product evolved through convergent evolution?
and finished their paper with these words:
Understanding the genes coding for biosynthetic pathways to lichen products, however, will be a major challenge for this century, one calling for strong collaboration between lichen chemists, systematists and molecular biologists
.
Lichen chemistry pages on this website Chemistry and taxonomy |
![An Australian Government Initiative [logo]](/images/austgovt_brown_90px.gif)

